PTK2 PROTAC
BI-3663
BI-3663 is a potent and selective PROTAC degrader of PTK2 kinase. This compound is a first-in-class low molecular weight degrader that harnesses the CRL4CRBN E3 ubiquitin ligase to trigger the intracellular destruction of PTK2 in a reversible but long-lasting manner. This compound is suitable for in vitro studies.
More information
Focal adhesion tyrosine kinase (PTK2) is a cytoplasmic protein tyrosine kinase that is overexpressed and activated in many types of advanced-stage solid cancers. PTK2 has been shown to play an important role in adhesion, spreading, motility, invasion, metastasis, survival, angiogenesis, epithelial to mesenchymal transition (EMT), cancer stem cells and the tumor microenvironment.2, 3 Overexpression and activation of PTK2 is associated with several human malignant diseases, and is correlated with poor overall patient survival.4-6 The focal adhesion tyrosine kinase (PTK2) is often over-expressed in human hepatocellular carcinoma (HCC) and several reports have linked PTK2 depletion and/or pharmacological inhibition to reduced tumorigenicity.7, 8 However, the clinical relevance of targeting PTK2 remains to be proven. Traditionally small molecules have been used to inhibit the action of a target protein by occupying and blocking a functional region of the protein. However, the disconnect between modulation of intracellular PTK2 autophosphorylation and growth inhibition as well as the often suboptimal selectivity profile of the inhibitors used makes it difficult to link the reported blockade of HCC tumour initiation and maintenance to PTK2 inhibition.
An alternative innovative approach is the development of proteolysis targeting chimeras (PROTACs), i.e. hetero bifunctional compounds consisting of one moiety that binds a Cullin RING E3 ubiquitin ligase linked to another that binds a desired protein of interest (POI), bringing the ligase and the POI into close spatial proximity This hijacks the intrinsic catalytic activity of the E3 ligase and directs it toward the POI as a neo-substrate, triggering its poly-ubiquitination and subsequent proteasome-dependent degradation. As a result, a PROTAC acts as a degrader of the target as opposed to just an inhibitor, enabling the effective post-translational elimination of a target gene product in living organisms.9 This approach presents many advantages compared to conventional target inhibition. One of the most attractive features of the approach is that a PROTAC molecule acts sub-stoichiometrically, i.e. it only needs to bind a molecule of target once to induce its degradation, and then is released and set free to bind another molecule of target and carry on, as in a catalytic cycle. For this reason, the concentrations required for PROTACs to be active in cells tend to be much lower compared to those needed to be reached and maintained with inhibitors, which can lead to fewer off-target effects and a more selective chemical intervention on the desired target.
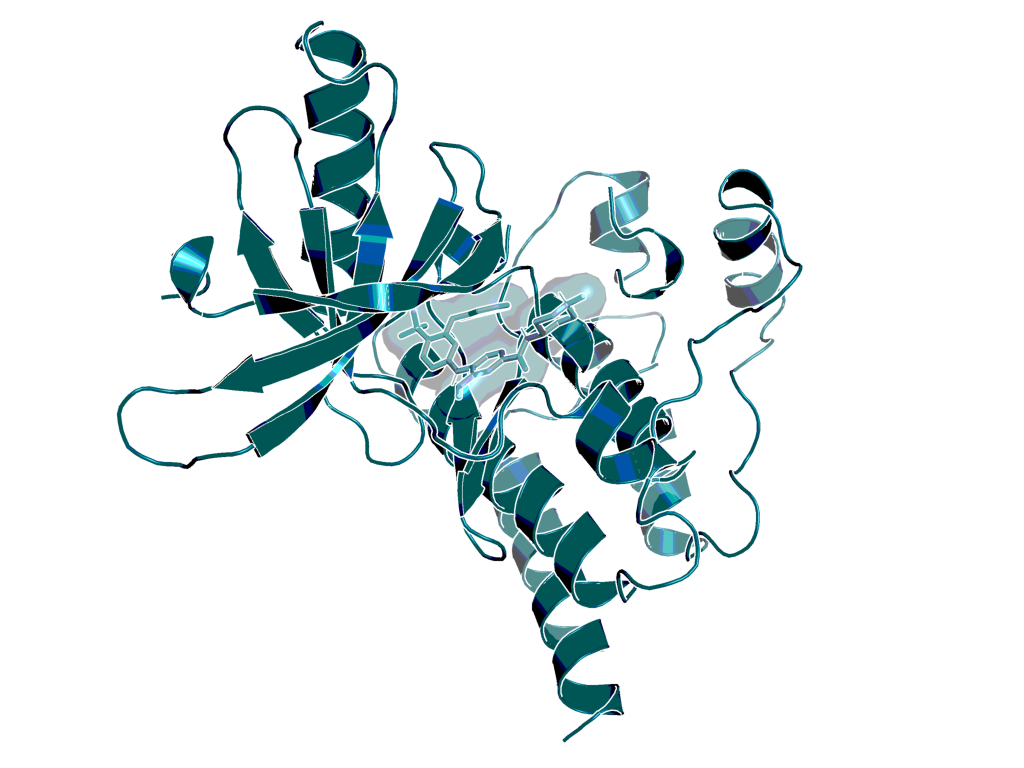
Structure of BI-4464 (ligand in BI-3663) bound to PTK2 (PDB ID 6I8Z). Hydrogen bonds to cysteine 502 and aspartic acid 564 are depicted in blue.
The binary affinities for BI-3663 to PTK2 and to CRBN (cereblon (CRBN) complex CRL4CRBN) are 18 and 877 nM respectively. BI-3663 degrades the PTK2 protein in A549 cells with a DC50 of 25 nM (Table 1). BI-3663 (cereblon-based) degrades PTK2 with a median DC50 of 30 nM to > 80% across a panel of eleven HCC cell lines (Table 2).
Table 1. Binary affinities of BI-4464 (PTK2 inhibitor) and BI-3663 for PTK2 and the respective PTK2 degradation data in A549 cells.
| BI-4463 (PKT2 inhibitor) | BI-3663 (PTK2 degrader) |
| E3 ligase ligand | - | POMA |
| PTK2 pIC50* | 7.8 ± 0.1 | 7.7 ± 0.1 |
| CRBN-DDB1 TR-FRET pIC50 | <4 | 6.1 |
| A549 cells, 18h, pDC50** | <4 | 7.6 ± 0.1 |
| A549 cells, 18h, Dmax[%]** | - | 95 ± 4 |
* Thermo Fisher SelectScreen Kinase Profiling Services, Z´-Light, ATP@Km, pIC50 ± STDEV
** Degradation activity is reported as concentration needed to achieve 50% PTK2 protein degradation (pDC50 ± STDEV) and maximal achievable protein degradation (Dmax) relative to DMSO. PTK2 levels were determined by protein capillary electrophoresis and normalized to GAPDH (N = 3).
Table 2. Degradation characteristics and effect on proliferation of BI-3663 and BI-4464 in HCC lines.
Cell Line | BI-4463 (PKT2 inhibitor) | BI-3663 (PTK2 degrader) | ||||
pDC50 | Dmax [%] | pIC50 (proliferation) | pIC50 (proliferation) | |||
| SNU-387 | 7.6 | 90.0 | <4.6 | 5.2 | ||
| HUH-1 | 6.6 | 50.0 | 4.6 | 5.4 | ||
| Hep3B2.1-7 | 7.9 | 96.0 | 4.6 | 5.3 | ||
| HepG2 | 7.5 | 89.0 | <4.6 | 5.5 | ||
| SK-Hep-1 | 7.5 | 89.0 | 5.8 | 5.2 | ||
| HLF | 6.4 | 30.0 | <4.6 | 5.4 | ||
| SNU-398 | 8.5 | 95.0 | <4.6 | 5.2 | ||
| HUCCT1 | 7.9 | 90.0 | <4.6 | 5.2 | ||
| HLE | 6.8 | 79.0 | <4.6 | 5.1 | ||
| HuH-7 | 7.3 | 93.0 | <4.6 | 5.4 | ||
| SNU-423 | 7.9 | 93.0 | 4.7 | 5.4 | ||
*Please note that data are reported as pIC50 which is calculated from IC50 and therefore have no unit.
BI-3663 is poorly soluble at physiological pH, and is a PGP substrate (high Caco2 efflux ratio). Of note, the CRBN-based PROTAC BI-3663 was considerably less stable in cell assay buffer containing 10% FCS (M+18 and +32 observed) than the VHL-based PROTAC BI-0319. BI-3663 was found to be stable as a solid and in DMSO stock solution (> 3 months, data not shown). Despite this previously reported instability of CRBN based PROTACs BI-3663 showed comparable maximal degradation of PTK2 after 18h and 72h days incubation.
| Probe name / negative control | BI-3663 | BI-4206 |
| MW [Da] | 918 | 1061 |
| Solubility @ pH 6.8 [µg/ml] | <1 | <1 |
| CACO permeability @pH7.4 [*10-6 cm/s] | 17 | 0.4 |
| CACO efflux ratio | 23 | 61 |
| Microsomal stability (human/mouse/rat) [% QH] | >95 / 95 / 95 | >95 / 90 / 54 |
| Half-life (EMEM, 10 % FCS) [h] | 13 | - |
| Plasma protein binding (human/mouse/rat/10% FCS) [%fu] | >99.4 / 99.5 / 99.7 / 88.7 | >99.8 / 99.9 / 99.8 / - |
cisVHL, BI-4206 is the (S) hydroxy diastereoisomer of BI-0319 and can also serve as a negative control for BI-3663. While exhibiting BI-3663 and BI-0319 comparable PTK2 binding affinity it no longer is able to bind and recruit VCB and therefore is not degrading PTK2 proteins in cells.
cisVHL — BI-4206 serves as a negative control.
In a 397 kinase kinase panel BI-0319 beside PTK2 only inhibited LRRK2 and FES by more than 50% at 1 µM. Interestingly, BI-0319 is more selective than the already highly selective PTK2 TKI BI-4464. We assume that the kinase selectivity panel for BI-3663 is comparable to BI-0319 since both exit vector and linker are identical for a distance of nine atoms from the piperidine moiety of the PTK2 ligand.
Multiplexed isobaric tagging mass spectrometry was employed to assess the cellular selectivity of BI-3663 for PTK2 degradation and identify potential degradation off-targets in a quantitative and unbiased manner. Amongst the 6,008 proteins quantified in this analysis in A549 cells, PTK2 showed a distinct and significant change in abundance upon treatment with BI-3663. BI-3663 did not induce any significant changes in abundance of other detectable kinases, thus confirming the high selectivity of both degraders within the kinase family. Of note, the two most prominent kinase off-targets of the inhibitor (LRRK2 and FES) were not detected in this dataset.
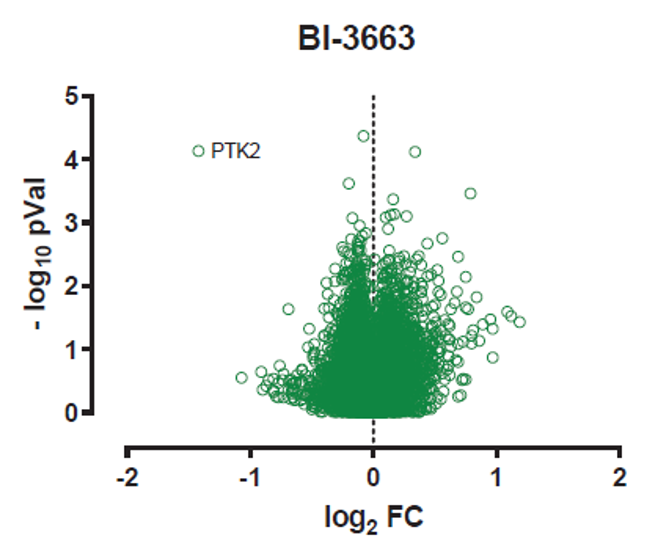
Total proteome analysis of A549 cells treated with BI-3663 for 18h and compared to DMSO controls. Samples were run in biological triplicates and analyzed by mass spectrometry. Volcano plot displays log2 of fold-change in abundance versus –log10 of adjusted p value (N = 3).
| SELECTIVITY DATA AVAILABLE | BI-3663 | BI-4206 |
SafetyScreen44™ with kind support of  | Yes | Yes |
| Invitrogen® | Yes | No |
| DiscoverX® | No | No |
| Dundee | No | No |
The PROTAC BI-3663 was tested by the Eric Fisher Laboratory - Dana-Farber Cancer Institute as part of their Degradation Proteomics Initiative.11,12 It induces selective degradation of PTK2 after 5 h of treatment at 3 µM in the neuroblastoma cell line Kelly Cells.

Global protein quantification was used to explore the unbiased proteome-wide selectivity of BI-3663 induced degradation. Whole cell protein quantification was performed using label free quantification with the Fischer lab’s diaPASEF workflow. Of the 7,742 proteins quantified in this experiment, only PTK2 was found to be significantly downregulated in response to BI-3663 treatment. Statistical analysis was performed using a moderated t-test in Bioconductor’s limma package to generate hit lists containing log2 Fold Change and P-values for each protein. The data are also displayed in the scatterplot above.
Download selectivity data:
BI-3663_selectivityData.xlsx
BI-4206_selectivityData.xlsx
Degradation_Proteomics_Initiative_data_BI-3663-PTK2-PROTAC_1.xlsx
There are currently no PTK2 PROTACs with the benchmarked selectivity
BI-3663 is a potent and selective PROTAC (proteolysis-targeting chimera) aimed at triggering the intracellular destruction of the PTK2 protein.
Highly Selective PTK2 Proteolysis Targeting Chimeras to Probe Focal Adhesion Kinase Scaffolding Functions
Popow J., Arnhof H., Bader G., Berger H., Ciulli A., Covini D., Dank C., Gmaschitz T., Greb P., Karolyi-Ozguer J., Koegl M., McConnell D. B., Pearson M., Rieger M., Rinnenthal J., Roessler V., Schrenk A., Spina M., Steurer S., Trainor N., Traxler E., Wieshofer, C. Zoephel A., Ettmayer P.
J. Med. Chem. 2019, 62, 2508-2520.
Focal adhesion kinase depletion reduces human hepatocellular carcinoma growth by repressing enhancer of zeste homolog 2
Gnani D., Romito I., Artuso S., Chierici M., De Stefanis C., Panera N., Crudele A., Ceccarelli S., Carcarino E., D'Oria V., Porru M., Giorda E., Ferrari K., Miele L., Villa E., Balsano C., Pasini D., Furlanello C., Locatelli F., Nobili V., Rota R., Leonetti C., Alisi A.
Cell Death Differ 2017, 24, 889-902.
Catalytic in vivo protein knockdown by small-molecule PROTACs
Bondeson D. P., Mares A., Smith I. E., Ko E., Campos S., Miah A. H., Mulholland K. E., Routly N., Buckley D. L., Gustafson J. L., Zinn N., Grandi P., Shimamura S., Bergamini G., Faelth-Savitski M., Bantscheff M., Cox C., Gordon D. A., Willard R. R., Flanagan J. J., Casillas L. N., Votta B. J., den Besten W., Famm K., Kruidenier L., Carter P. S., Harling J. D., Churcher I., Crews C. M.
Nat Chem Biol 2015, 11, 611-617.
The data can be accessed via the following link:
Mapping the Degradable Kinome Provides a Resource for Expedited Degrader Development
Donovan K. A., Ferguson F. M., Bushman J. W., Eleuteri N. A., Bhunia D., Ryu S., Tan L., Shi K., Yue H., Liu X., Dobrovolsky D., Jiang B., Wang J., Hao M., You I., Teng M., Liang Y., Hatcher J., Li Z., Manz T. D., Groendyke B., Hu W., Nam Y., Sengupta S., Cho H., Shin I., Agius M. P., Ghobrial I. M., Ma M. W., Che J., Buhrlage S. J., Sim T., Gray N. S., Fischer E. S.
Cell. 2020, 183(6):1714-1731.
When you plan a publication, please use the following acknowledgement:
BI-3663 was kindly provided by Boehringer Ingelheim via its open innovation platform opnMe, available at https://www.opnme.com.