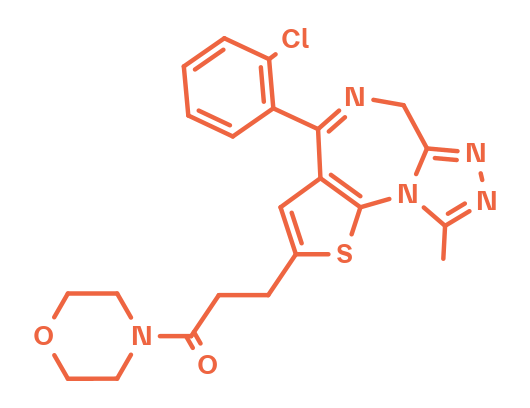
Apafant
Apafant WEB2086
Apafant is a potent and specific synthetic antagonist of the pro-inflammatory platelet activating factor (PAF) receptor. It is employed for the in vitro and in vivo study of the PAF pathway. The PAFR antagonist Bepafant, and its active enantiomer, S-Bepafant are also provided. The structurally related WEB2387 is used as negative control.
More information
The platelet-activating-factor receptor (PAFR) is a G-protein-coupled seven-transmembrane receptor that plays a profound role in stimulating inflammatory and thrombotic responses. PAFR is activated by platelet-activating-factor (PAF), which comprises a family of structurally related agonistic phospholipids that bind with high affinity to the receptor. PAFR stimulation mediates numerous cellular responses such as activation of the mitogen-activated protein kinase (MAPK) pathway, phosphoinositol turnover, platelet and granulocyte aggregation, and chemotaxis of leukocytes. PAF levels are elevated in disease tissues and fluids that lead to, amongst others, systemic hypotension, increased vascular permeability and thrombocytopenia. The interest in PAFR as a therapeutic target by inhibiting its function is underlined by its association with over 40 disease states that range from asthma to cancer. A number of diverse antagonists and inverse agonists of PAFR have been described that are either based on the original phospholipid structures or natural products, or entirely novel synthetic scaffolds. Apafant represents a potent and well-characterised member of the latter class3,6,7,8.
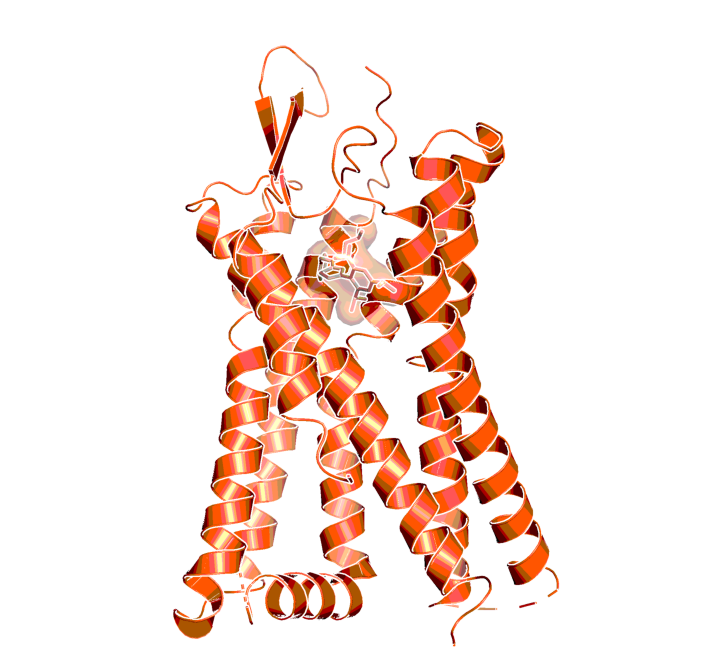
PAF receptor in complex with the ligand SR 27417, indicating the presumed binding location of Apafant, as determined by X-ray crystallography (PDB code 5ZKP14)
Apafant binds with high affinity to the PAF receptor on human platelets, as determined by displacement of the natural ligand PAF from the PAFR receptor complex. Moreover, PAF-induced aggregation of both human platelets and neutrophils is inhibited by Apafant in a dose-dependent manner. The interaction is specific as neither Apafant or Bepafant have significant effects on platelet or neutrophil aggregation in response to other aggregating agents1,11. Despite the structural similarity of thienotriazolodiazepines to the CNS-acting benzodiazepines, Apafant shows only modest cross-reactivity to the central benzodiazepine receptor2. This activity is attenuated further (10-fold) in the otherwise equally potent Bepafant. Both compounds display relatively low partition coefficients (logD, see below) resulting in low brain exposure2, and importantly, benzodiazepine-like effects were not observed at high doses in humans4. In competition experiments with [3H]PAF, Apafant displaces the natural ligand PAF with an equilibrium dissociation constant (KD) of 15 nM, thereby inhibiting the signaling function of PAFR. PAF-induced human platelet and neutrophil aggregation is inhibited in vitro at lC50’s of 170 and 360 nM, respectively.
| APAFANT | BEPAFANT | S-BEPAFANT | NEGATIVE CONTROL WEB2387 |
| MW [Da]a | 456.0 | 468.0 | 468.0 | 468.0 |
| Receptor Binding (KD) [nM], humanb | 152 | 169 | 149 | 6609 |
| Platelet aggregation (IC50) [nM], humanc | 1701,11 | 3109,11 | 3509 | 87909 |
| Neutrophil aggregation (IC50) [nM], humand | 3601 | 83010 | n.a. | n.a. |
| Benzodiazepine receptor inhibition (Ki) [nM], rate | 3882 | 34952 | n.a. | n.a. |
aFor the salt form you will get, please refer to the label on the vial and for the molecular weight of the salt, please refer to the FAQs
bTritiated [3H]PAF binding to human platelets was inhibited by addition of increasing concentrations of Apafant, from which the KD was determined. In a reverse experiment, [3H]Apafant was displaced by PAF and Apafant to the same degree. Refer to respective references for detailed methods.
cPlatelet-rich plasma isolated from human venous blood was collected, and aggregation was induced by addition of PAF. The aggregation inhibitory effect of the antagonists was determined adding various concentrations to the reaction mixture one minute prior to the addition of PAF. Refer to respective references for detailed methods.
dHuman leukocytes were isolated from human venous blood. Aggregation was induced by addition of PAF, and the aggregation inhibitory effect of the antagonists was determined adding various concentrations to the reaction three minutes prior to the addition of PAF. Refer to respective references for detailed methods.
eSelectivity to benzodiazepine receptors was tested through inhibition of [3H]flunitrazepam binding to rat cortex synaptosomal membranes as a function of PAF antagonist concentration. Refer to respective references for detailed methods.
| APAFANT | BEPAFANT | S-BEPAFANT | NEGATIVE CONTROL WEB2387 | |
| Solubility at pH 2.0/6.8 [µg/ml] | 55 / >100 | 33 / >100 | 51 / >100 | 44 / 86 |
| logD @ pH2 / pH11 | 1.08 / 1.12 | 1.21 / 1.15 | 1.2 / 1.14 | 1.18 / 1.12 |
| Plasma Protein Binding (%) human/rat | degradation / 65 | 54 / 33 | 38 / 34 | n.d. / n.d. |
| Caco-2 permeability AB @ pH 7.4 [*10-6 cm/s] | 3.2 | 11.8 | 7.1 | 15.1 |
| Caco-2 efflux ratio | 14.5 | 6.4 | 4.9 | 6.8 |
| Microsomal stability (human/rat) [% QH] | 24.9 / 38.3 | <23 / 25.4 | <23 / 24.3 | <23 / 25.1 |
| MDCK permeability Pappa-b/b-a @ 1µM [10-6 cm/s] | 0.25 | 1.1 | 0.94 | 0.72 |
| MDCK efflux ratio | 7 | 20.9 | 25.5 | 43.1 |
| Hepatocyte stability (human/rat) [% QH] | 20 / 54 | 7 / 55 | <4 / 48 | 6 / 58 |
| CYP 3A4 (IC50) [µM] | >50 | n.d. | >50 | n.d. |
| CYP 2D6 (IC50) [µM] | >50 | n.d. | >50 | n.d. |
| CYP 2C8 (IC50) [µM] | >50 | n.d. | >50 | n.d. |
| CYP 2C9 (IC50) [µM] | >50 | n.d. | >50 | n.d. |
| CYP 2C19 (IC50) [µM] | >50 | n.d. | >50 | n.d. |
| CODE | APAFANT | BEPAFANT | S-BEPAFANT |
| tmax [h] rat | 0.3a | 0.8b | n.d. |
| Cmax [nM] rat | 449 a | 491 b | n.d. |
| Clearance [ml/(min*kg)]] | n.d. | 76 c | 44 d |
| Mean residence time after i.v. dose [h] rat | n.d. | 0.38 | 0.5 |
| F [%] | n.d. | 37b | n.d. |
| VSS [l/kg] | n.d. | 1.7c | 1.3 |
| t1/2 [h], rat | 3.1 | 5.4b | n.d. |
ap.o. dose: 5.3 mg/kg
bp.o. dose: 5.0 mg/kg
di.v. dose: 0.48 mg/kg
di.v. dose: 1.0 mg/kg
Acute bronchoconstriction induced by intravenously administered PAF is widely used to characterise PAF antagonists in animal models, where the antagonist efficacy is quantified by determining the recovery of respiratory flow and mean arterial pressure (MAP, a measure of hypotension).
In vivo, extensive investigations using a range of animal models of human disease showed Apafant to potently reduce bronchoconstriction, hypotension, microvascular leakage, and anaphylactic shock amongst many others1,2,3,13.
Apafant displays an ED50 of 0.07 and 0.018 mg/kg in guinea pigs when administered orally and intravenously, respectively, and the ED50 for MAP is comparable. Despite the similar in vitro properties (see above), Bepafant displays enhanced potency (ED50 of 0.016 mg/kg for respiratory flow)10. This is likely caused by the increased t1/2 for Bepafant (see table above)11. The eutomer of Bepafant (S-Bepafant) shows an additional slight increase in potency compared to the racemic Bepafant, while the distomer (WEB2387, negative control) shows a 40-80-fold reduction of in vivo potency compared to S-Bepafant12.
| PROBE NAME / NEGATIVE CONTROL | APAFANT | BEPAFANT | S-BEPAFANT | NEGATIVE CONTROL WEB2387 |
| Respiratory flow ED50 [mg/kg] p.o. | 0.07 | 0.021 | 0.018 | 1.55 |
| Respiratory flow ED50 [mg/kg] i.v. | 0.018 | 0.007 | 0.004 | 0.081 |
| Mean arterial pressure ED50 [mg/kg] p.o. | 0.066 | 0.02 | 0.027 | 1.2 |
| Mean arterial pressure ED50 [mg/kg] i.v. | 0.016 | 0.006 | 0.005 | 0.086 |
Further studies showed that Apafant inhibits PAF-induced vascular leakage (as measured by the extravasation of Evans blue dye) fully at 10 mg/kg i.v. in the guinea pig.
Antigen-induced anaphylactic shock and bronchoconstriction was prevented by both Apafant and bepafant in guinea pigs co-treated with the antihistamine mepyramine, with 1.0 mg/kg bepafant p.o. providing almost complete protection.
In a model of inflammation, both Apafant and bepafant significantly attenuated PAF-induced paw edema in the rat, with Bepafant showing greater potency in this model.
Various additional pharmacology studies are reviewed in reference 2.
WEB2387 is offered as a negative control. It is the distomer (inactive enantiomer) of active, racemic Bepafant. Thus, WEB2387 is an appropriate negative control for Bepafant and S-Bepafant, and the structurally related Apafant.
WEB2387 which serves as a negative control
The SafetyScreen44™ panel have been measured for all four compounds and it showed no relevant off-target effects.
| SELECTIVITY DATA AVILABLE | APAFANT | BEPAFANT | S-BEPAFANT | NEGATIVE CONTROL WEB2387 |
SafetyScreen44™ with kind support of  | Yes | Yes | Yes | Yes |
| Invitrogen® | No | No | No | No |
| DiscoverX® | No | No | No | No |
| Dundee | No | No | No | No |
Download selectivity data:
WEB2086BS_selectivityData.xlsx
WEB2387BS_selectivityData.xlsx
Apafant is a potent and specific synthetic antagonist of the pro-inflammatory platelet activating factor (PAF) receptor since its first disclosure in 1987. It is employed for the in vitro and in vivo study of the PAF pathway, and has been investigated in clinical studies for indications such as asthma4,5. It has been investigated in a range of disease models ranging from inflammatory disorders to cancer. The PAFR antagonist Bepafant, and its active enantiomer, S-Bepafant are also provided. The structurally related WEB2387 is used as negative control.
Pharmacological actions of WEB 2086, a new specific antagonist of platelet activating factor
Casals-Stenzel J, Muacevic G, Weber KH
J Pharmacol Exp Ther 1987, 241, (3), 974-81.
Pharmacodynamics, pharmacokinetics and safety profile of the new platelet-activating factor antagonist apafant
Brecht HM, Adamus WS, Heuer HO, Birke FW, Kempe ER
man. Arzneimittelforschung 1991, 41(1):51-9.
Platelet Activating Factor Antagonists
Summers JB, Davidsen SK, Sheppard GS
Current Pharmaceutical Design 1995, 1, 161-190.
Biological characterization of the enantiomeric hetrazepines of the paf-antagonist web 2170
Heuer H, Birke F, Brandt K, Muacevi G, Weber KH
Prostaglandins 1988, (35)5, 847.
Pharmacologic activity of bepafant (WEB 2170), a new and selective hetrazepinoic antagonist of platelet activating factor
Heuer HO, Casals-Stenzel J, Muacevic G, Weber KH
J Pharmacol Exp Ther 1990, 255(3):962-8.
Thieno-traizolo-1,4-diazepino-2-carboxylic acid amides, process for their preparation and pharmaceutical compositions (EP194416)
Weber, Karl-Heinz; Harreus, Albrecht; Stenzel, Jorge C.; Troger, Wolfgang; Walther, Gerhard; Muacevic, Gojko
1986
When you plan a publication, please use the following acknowledgement:
PAF Receptor Antagonist was kindly provided by Boehringer Ingelheim via its open innovation platform opnMe, available at https://www.opnme.com.